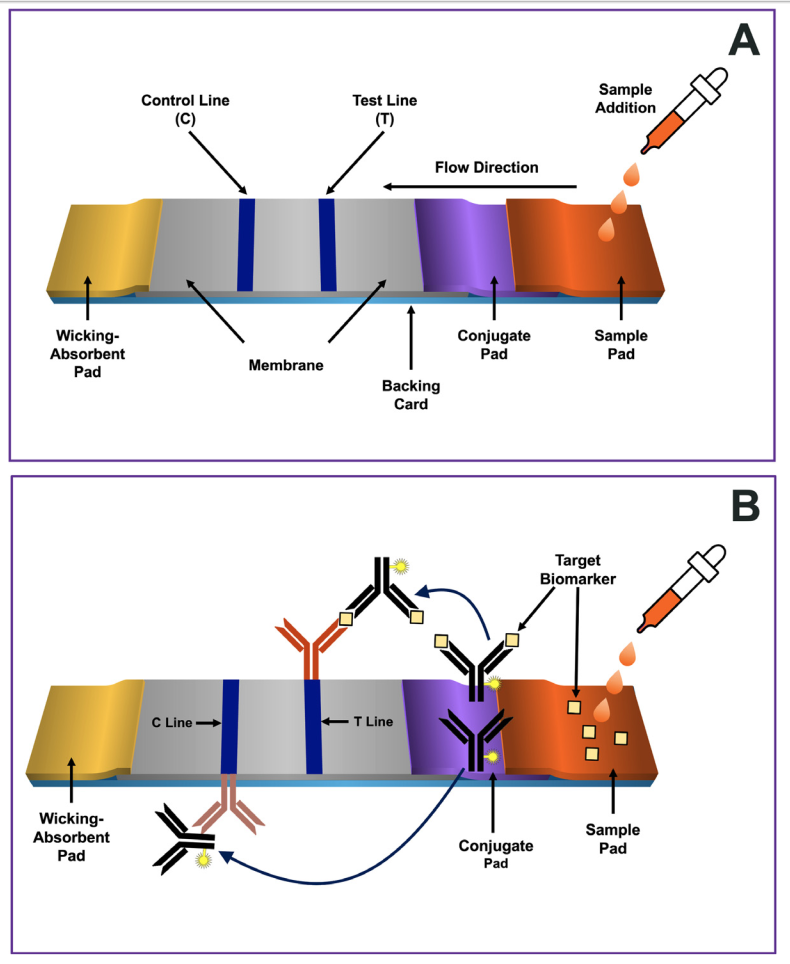
Fig. 1. Schematic representation of a common three antibody format of a lateral flow immunoassay.
Panel A depicts four key design components forming the platform:
a sample pad, a conjugate pad containing labeled antibodies (e.g., antibody-labeled conjugate),
detection membrane with immobilized antibodies at the T and C-lines,
and an absorbent pad. The components of the strip are usually fixed to an inert material backing card,
to maintain the orientation of the four components’ pads to assure adequate flow across them.
Panel B depicts the target biomarker and the three antibodies used in the sandwich format. There are two antibodies specific for the target biomarker.
The first, referred to as the conjugate, serves as the “detection” component of the test. It is an antibody-labeled conjugate, meaning the antibody is covalently coupled with a signal producing label, which in this example generates a colored line (black antibody).
The second antibody (orange antibody) is specific for a different epitope on the
target biomarker and is immobilized in the T-line. This immobilized antibody become bridged with the conjugate by the target biomarker, forming a “sandwich” interaction that is visual at the T-line if the target biomarker is present.
The third antibody (brown color) is immobilized at the control line and is directed against the conserved region (Fc) of the conjugate antibody. The conjugate antibody that did not bind with the target biomarker flows past the T-line and is captured at the C-line.
As with the T-line, the conjugate’s label enables the visualization of the line. The sandwich complex that results in a visible T-line is contingent on the presence of the target biomarker.
A visible T-line indicates a positive test. If the sandwich complex does not form, no visual line will be formed, and the outcome is interpreted as a negative result. However, for the LFIA to be valid, the C line must be visible as well. If a color is formed at the C-line, it means that the LFIA test functioned properly. If not, the result is considered invalid or not conclusive. Figure modified from Refs. [22,55,57,58].
Benefits of LFIA
provides rapid results within minutes, enabling healthcare providers to make timely decisions and provide immediate care to patients.
easy to use and do not require specialized equipment or highly trained personnel, making them a valuable tool for use in resource-limited settings and remote areas.
that many tests require only a small sample, such as a drop of blood, making them less invasive and more comfortable for patients.
portability; allowing performance in the field without the need for a laboratory or complex infrastructure, making them ideal for use in emergency situations, disaster response scenarios, and other situations where rapid and accurate diagnoses are critical.
The cost-effectiveness of LFIA tests further underscores their value in clinical diagnostic. Compared to traditional laboratory based tests, LFIA tests are substantially less expensive, making them more accessible and affordable for both patients and healthcare providers. LFIA tests also have the potential to reduce healthcare costs by providing early and accurate diagnoses, leading to more targeted treatments, as well as fewer and unnecessary tests, that often require invasive procedures.
been approved for use in detecting a variety of diseases and disease states, providing patients with quick and reliable diagnostic information while also reducing the burden on the healthcare system.
have been approved by the U.S. Food and Drug Administration (FDA) for use in the detection of a range of pathogens, including HIV, hepatitis B and C, influenza, and COVID-19 [22-31,58].
been approved for use in the diagnosis and monitoring of chronic diseases, such as diabetes and heart disease. For chronic diseases like diabetes and heart disease, early detection can also help patients better manage their condition and prevent further complications.
Limitations of LFIA
limited analytical sensitivity [66–70], which may lead to false-negative outcomes.
specificity is compromised by potential cross-reactivity with similar biomolecules (e.g.,isoenzymes, proteoforms, metabolites) giving rise to false-positive results.
not suitable for all sample types, particularly those that are highly viscous or complex such as sputum that may need a sample pretreatment process before use [22].
provides only qualitative results, categorizing them as “positive” or “negative” without offering detailed measurement of the target biomarker [73].
limited to the detection of a single target biomolecule and are unable to quantify multiple biomolecules simultaneously. This can limit their usefulness in monitoring disease progression or treatment effectiveness [76].
Due to above limitations, LFIA used primarily for initial screening tests, to be backed up by further cpnfirmational assays.
